Carbon Dioxide Control – The Must & The Path

Abnormal climate change and uncontrolled natural calamities and catastrophes are increasing in the recent years. The overwhelming majority of scientists agree that our globe is undergoing major climate change. They also agree that the level of carbon dioxide in the atmosphere is rising significantly. There is a gradual increase in the average temperature of the Earth’s atmosphere in the last 100 years. The CO2 concentration in the atmosphere is increased due to the continued in balance of its production and consumption. The reduction of CO2 is significantly affected by deforestation. Natural fixation of CO2 in the atmosphere such as photosynthesis and ocean carbon cycle is not enough to control the growing amount of CO2. Artificial fixation of CO2 is mandated. Carbon dioxide is captured and extracted by various methods. Techniques using effective absorber, conventional and nano-catalytic methods, capturing using enzymes and capturing using microalgae methods are few pathways to reduce CO2. The turn over number of CO2 capture in the catalytic process is directly proportional to the surface area of the catalyst material and the nature of the active site of the catalyst. CO2 can be converted into useful chemicals such us methane, methanol, oxygen and biomass. The continued challenge of converting CO2 into useful material and the need for the large CO2 reduction using a little amount of reducing material is a growing research.
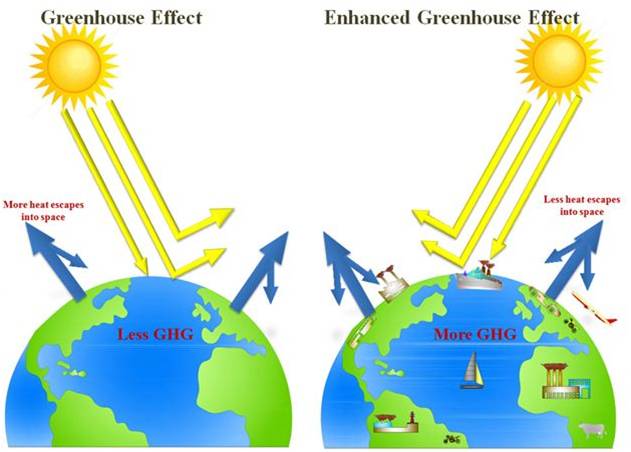
Enhanced Greenhouse Effect

Greenhouse gas (GHG) is a gas in an atmosphere that absorbs and emits radiation within the thermal infrared range. Greenhouse effect is one of earth’s natural processes to regulate the temperature of our planet. It is essential for life on earth and is unquestionably real. It is a result of heat absorption by greenhouse gases in the lower atmosphere and re-radiation downward of some of that heat. Greenhouse gases greatly affect the temperature of the Earth. The concern is not with the fact that we have a greenhouse effect, but whether human activities are leading to an enhancement of the greenhouse effect by the emission of more and more greenhouse gases through fossil fuel combustion and deforestation. Carbon dioxide from combustion of coal, oil and gas concentration is increasing at a rate of 1.9 ppm/year since 2000. The pre-industrial level of CO2 was about 280 ppm and the current level is about 380 ppm. Intergovernmental Panel on Climate Change (IPCC) projects the growth range from 490 ppm to 1260 ppm by the end of 21st century.
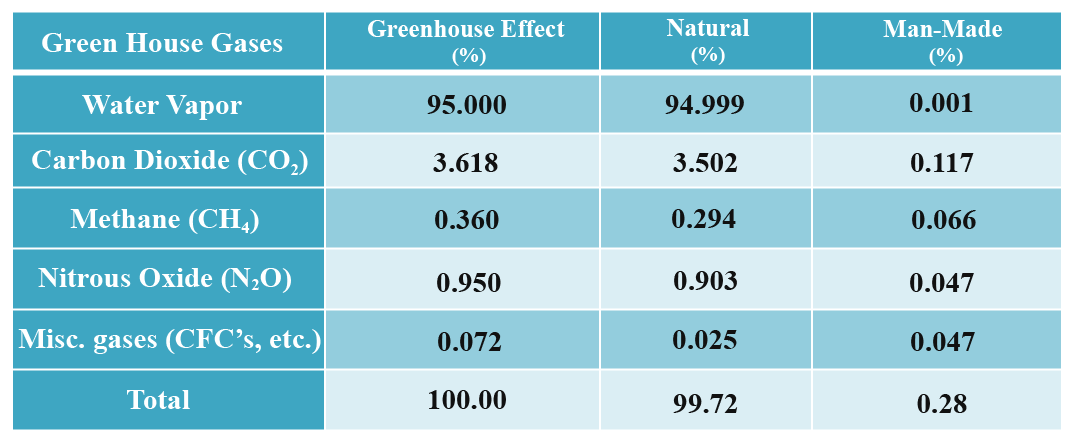
Greenhouse Gases and Contributions

The effect of greenhouse gas not only depends on the quantity but also on their chemical nature. For example, the influence of methane and CFC are very high though they are present in lower quantities. Though water vapor dominates the greenhouse effect among the contributing gases, CO2 plays a major role as it influences the increase of water vapor concentrations. The concentration of nitrous oxide began to rise at the beginning of the industrial revolution. Miscellaneous greenhouse gases such as CFC’s are mostly synthesized by the use of refrigerants, aerosol propellants and cleaning solvents.
Carbon Dioxide Balance


Approximately 600 bt of carbon moves back and forth between the atmosphere and the oceans and between the atmosphere and the land biosphere. Although these exchange rates are large relative to the total amount of carbon stored in the atmosphere, the concentration of 2200 bt CO2 was constant. Marine plants and animals play a role in the uptake and release of carbon dioxide in the ocean. Atmospheric concentrations of CO2 were constant because the carbon being removed from the atmosphere in some places exactly matched the CO2 being added to the atmosphere in other places. Most of the CO2 released from the burning of fossil fuels and other human activities is stored either in the atmosphere or in the oceans. The CO2 that remains in the atmosphere acts as a greenhouse gas, absorbing long-wavelength radiation (heat) in the atmosphere. CO2 taken up by the oceans does not affect the Earth’s heat balance. So, an understanding of the air-sea exchange of CO2 is an essential part of understanding the Earth’s climate system and the potential impact of future CO2 emissions.
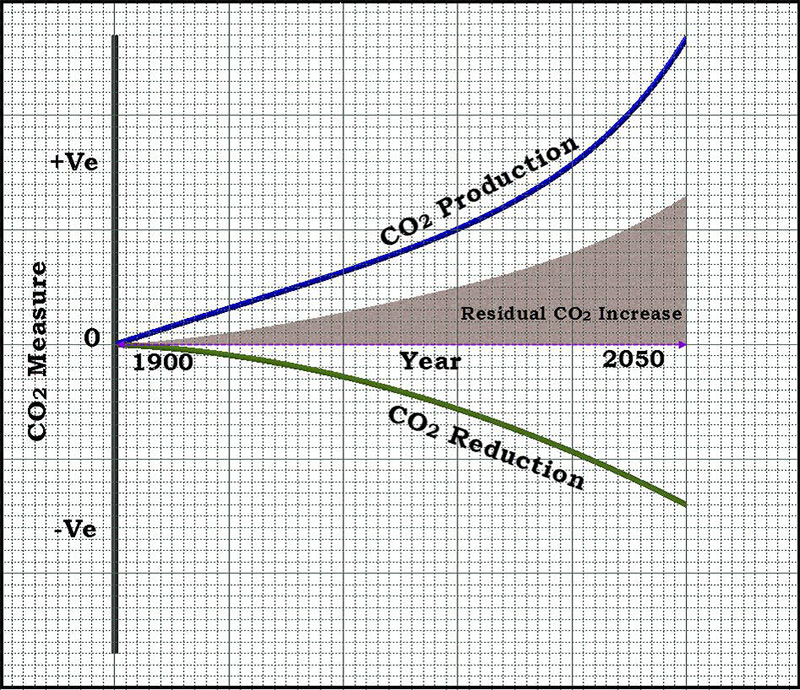
The Must & The Path

The rate of formation of CO2 and the rates of reduction of CO2 in the atmosphere are not proportional. In time reduction of CO2 is very much affected due to deforestation and increased human activities and the formation of CO2 increased abnormally due to industrial revolution. The formation of CO2 increases exponentially where as the reduction is not observed in the same rate. This leads to a growing gap between the two processes and the increase in the residual CO2 concentration in the atmosphere. The present society intends on achieving influences and a convenience has forgotten how precious nature is. Enhanced CO2 is a very big challenge we are faced with today. The impact of CO2 on human health can be of different extend starting from minor shortness of breath to fatal death. It is very clear that CO2 can cause damage to health and also responsible for enhanced greenhouse effect leading to global warming. Thus identifying the ways to reduce CO2 becomes critical. This demands the need for fixing the CO2 concentration in the atmosphere to avoid the impact of CO2 on human health, environment and societal welfare.
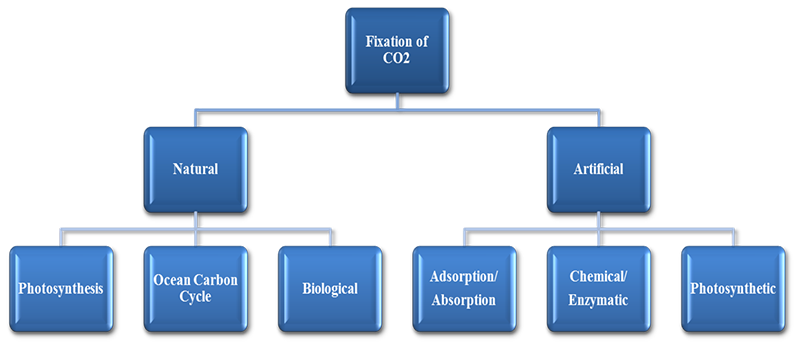
CO2 Fixation

Carbon fixation refers to the process by which gaseous carbon dioxide is captured, extracted, utilized and fixed. It refers mostly to the processes found in autotrophs (organisms that produce their own food), usually driven by photosynthesis, whereby carbon dioxide is changed into sugars. Carbon fixation can also be carried out by the process of calcification in marine calcifying organisms and also by heterotrophic organisms in some circumstances. Very limited CO2 fixation also occurs in nature using light and other sources such as by algae and enzymes by converting into organic carbon compounds and biomass.
Natural Fixation

Natural Ocean Carbon Cycle
The oceans contain about 50 times more CO2 than the atmosphere and 19 times more than the land biosphere. CO2 moves between the atmosphere and the ocean by molecular diffusion when there is a difference between CO2 gas pressure (PCO2) between the atmosphere and oceans. For example, when the atmospheric PCO2 is higher than the surface of the ocean, CO2 diffuses across the air-sea boundary into the sea water. The oceans are able to hold much more carbon than the atmosphere because most of the CO2 that diffuses into the oceans reacts with the water to form carbonic acid and its dissociation products, bicarbonate and carbonate ions. The conversion of CO2 gas into nongaseous forms such as carbonic acid and bicarbonate and carbonate ions effectively reduces the CO2 gas pressure in the water, thereby allowing more diffusion from the atmosphere. The oceans are mixed much more slowly than the atmosphere, so there are large horizontal and vertical changes in CO2 concentration. In general, tropical waters release CO2 to the atmosphere, whereas high-latitude oceans take up CO2 from the atmosphere. CO2 is also about 10 percent higher in the deep ocean than at the surface.
Photosynthesis
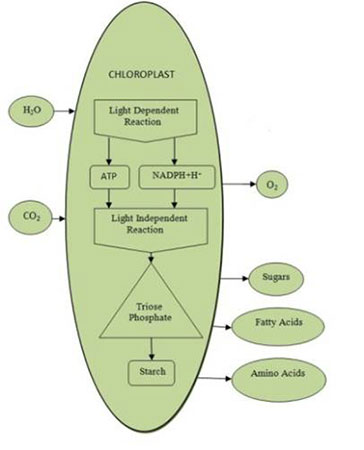
Photosynthesis is the process of producing organic molecules using the energy of light. The reaction for the production of glucose, the most abundant product and equation is given below:
6CO2 + 12H2O C6H12O6 + 6H2O + 6O2
Factors limiting photosynthetic rates include light intensity, water availability, soil nutrient content, concentration of carbon dioxide and temperature. The light dependent pathways produce ATP and NADPH+H+ to be used in the light independent processes. The compound requiring the NADPH+H+ and ATP was found to be Glycerate 3-phosphate (GP). GP is converted to various other three carbon sugars or Triose phosphates (TP), some are used to produce hexodes such as fructose phosphate and glucose phosphate.
Artificial Fixation

Fixation by Absorption
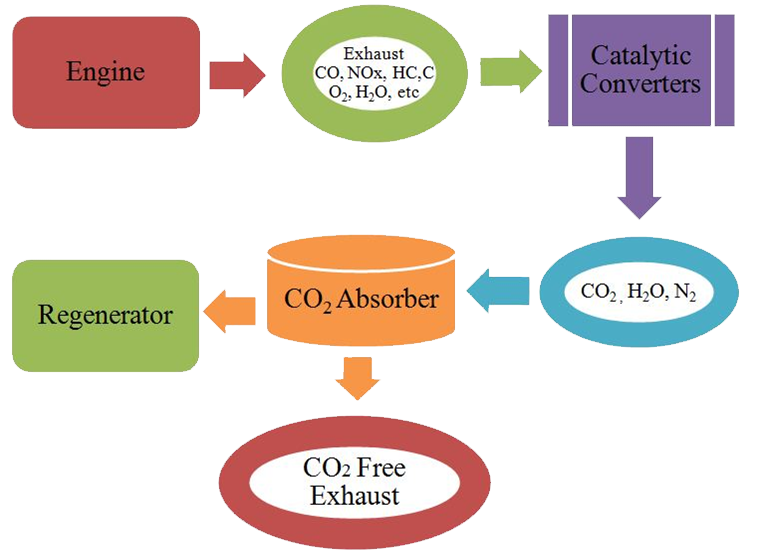
In principle, CO2 is reduced from the gas by absorbing it into source and regenerating the source into its original. Exhaust gas is emitted as a result of the combustion of fuels from the engine. The conventional catalytic converter promotes the conversion of hydrocarbon (HC), nitrous oxides (NOx) and carbon monoxide (CO) in the engine’s exhaust into carbon dioxide (CO2), nitrogen (N2) and water (H2O) vapor. The CO2 absorber will absorb the CO2 and then it will dispatch into CO2 free exhaust and the CO2 absorber is regenerated. Different types of sorbents are used depending upon the source and the need.
Amines as Sorbents
The simple method is to pass the gas through a chemical solvent that selectively absorbs only the CO2 and keeps it in a weak chemical bond. The CO2 releases from the chemical solvent during regeneration by heat addition that breaks the bond of the CO2. The almost pure CO2 is collected, compressed and sent to storage. The retrieved solvent goes for re-use. The most common chemical solvents used are amines. Amine capture is a proven system in the natural gas cleaning process. The scale of operations is much larger for the removal of CO2 from power plants. Even though many pilot plants are in service, a commercially viable operation on a large scale is yet to take place.
Lithium Hydroxide as Sorbent
Lithium Hydroxide (LiOH) is the most commonly used CO2 sorbent for use in expendable devices.
The presence of water vapor is important to the functioning of LiOH beds. Chemisorption of CO2 is thought to take place via a two step reaction in which lithium monohydrate is first formed by the exothermic reaction followed by the endothermic formation of lithium carbonate.

Capturing by Enzyme
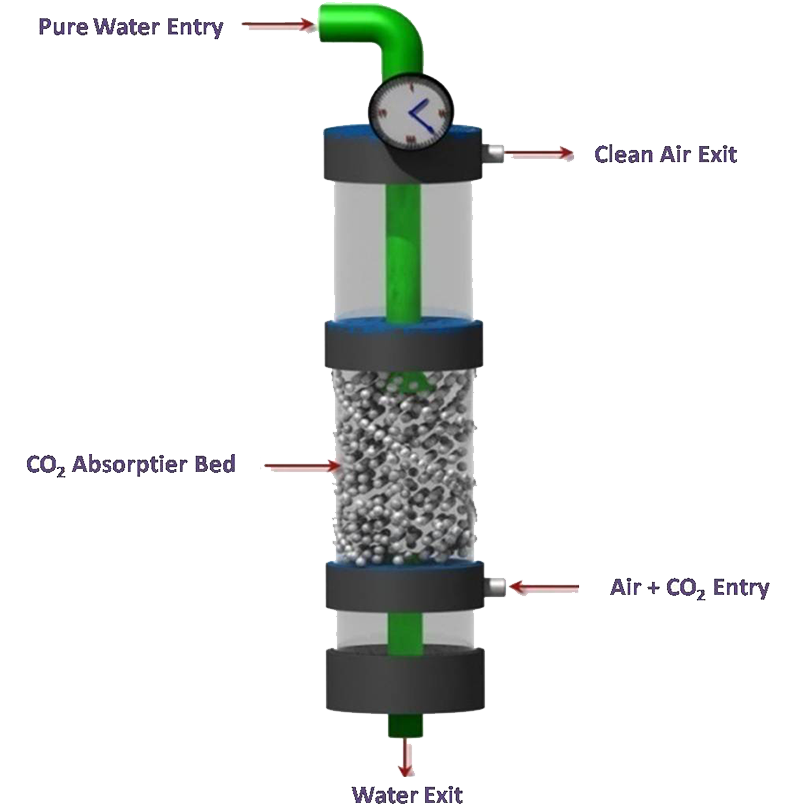
CO2 solution bioreactor contains an enzyme-bonded packing material that interacts with a water solution pumped in from the top and smokestack emissions that enter and bubble up through the bottom. The carbon dioxide in the emissions stream is captured by the enzymes on the surface of the packing material and converted into bicarbonate ions. Cleaned-up air then escapes from the top while the bicarbonate solution exits of the bottom. The bicarbonate, in a separate process, can be extracted from the solution and made into compounds, such as limestone, for use by industry. A new way to capture carbon dioxide from smokestacks produces a raw material that can be sequestered underground or turned into substances such as baking soda, chalk, or limestone. The bioreactor is a long cylinder containing a packing material that acts as a solid support for the enzyme. The surface of this material has been chemically modified so that the enzymes attach securely. At the top of the cylinder, a water solution is pumped in and flows around the packing material, while gases from a smokestack enter the bottom of the cylinder and bubble up through the solution. The carbon dioxide is absorbed into the solution and then interacts with the enzymes, which convert the greenhouse gas into bicarbonate ions. To end the process, cleaned up air escapes from the top while the bicarbonate solution is extracted for further processing either back into pure carbon dioxide for long-term geological storage or into a carbonate compound, such as limestone, that can be used by industry.
Fixation Using Microalgae
Microalgae have attracted a great deal of attention for CO2 fixation and biofuel production because they can convert CO2 into biomass via photosynthesis at much higher rates than conventional biofuel crops can. Microalgae-mediated CO2 fixation and biofuel production can be rendered more sustainable by coupling microalgae biomass production with existing power generation and wastewater treatment infrastructures. Microalgae can utilize low-quality water, such as agricultural runoff or municipal, industrial or agricultural wastewaters, as a source of water for the growth medium as well as a source of nitrogen, phosphorus and minor nutrients. Although the most common application of microalgae in wastewater treatment aims at nutrient removal/recovery, microalgae have also been utilized for removal of heavy metals and organic matter. Finally, secondary utilization of microalgae has been successful in toxicity monitoring.
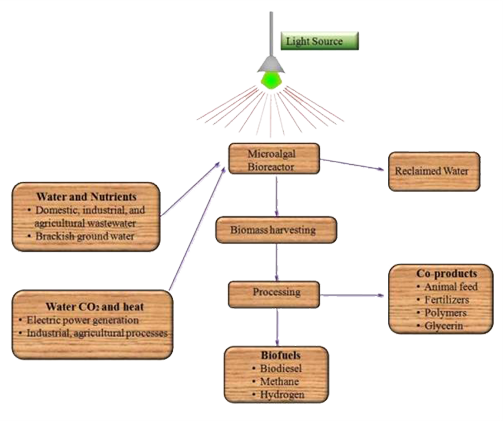
The combination of the three roles of microalgae CO2 fixation, wastewater treatment and biofuel production has the potential to maximize the impact of microalgae biofuel production systems, and has accordingly been investigated. However, a number of crucial research gaps remain that must be overcome to achieve full-scale operation including: (i) improved algal growth and nutrient uptake rates; (ii) integration of biosystems with waste gas, wastewater and water reclamation systems; (iii) improved gas transfer and mixing; (iv) improved algal harvesting and dewatering; and (v) life cycle analysis (LCA) and associated economic assessment. In addition, there is a lack of fundamental information needed to rationally optimize the performance of existing bioreactors. Novel bioreactor configurations and designs are also needed that promote microalgae growth, characterized by volumetric productivities at least one order of magnitude above those of conventional open pond facilities.
CO2 Fixation Using Catalyst
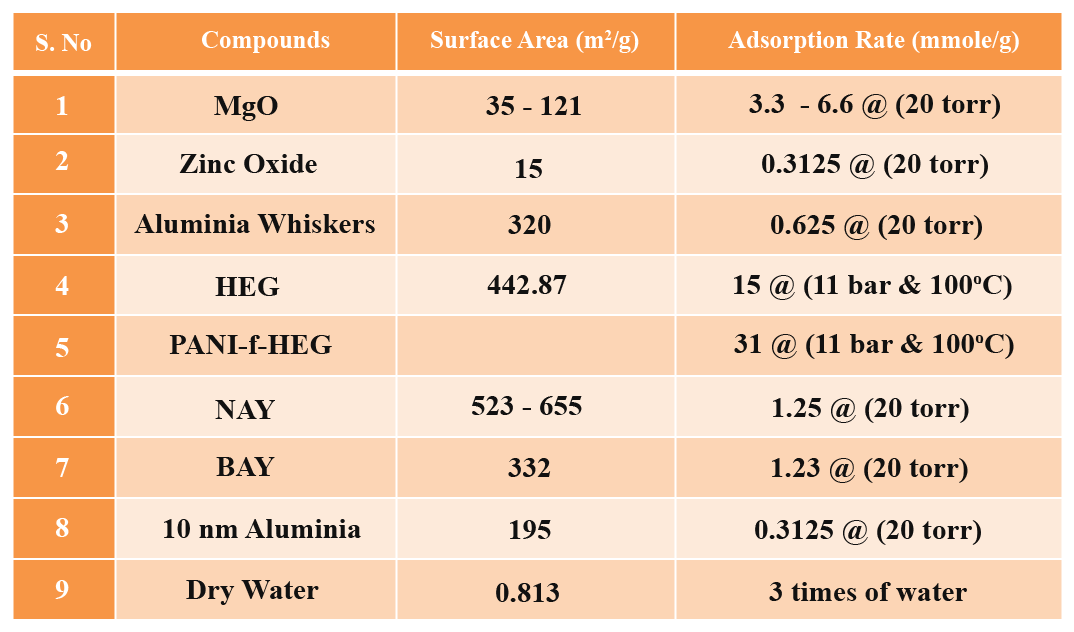
Conventional catalysts such as activated alumina, magnesium oxide, graphene, natural zeolites, clays and zinc oxide are used to reduce CO2. The efficiency of these compounds is not enough to reduce the large quantity of CO2 in the atmosphere. The turn over number, the number of molecules reacted per site per time is too low using these materials due to lower surface area and low activity. More effective catalysts with larger surface area and efficient active sites need to be developed. Nano-catalysts are one such material providing higher activities. i.e., its incorporation in organic molecules such as formic acid requires a catalyst. Bulk CdS and ZnS surfaces catalyze CO2 fixation in the presence of light, but CdSe surfaces do not. However, Cd-rich CdSe nanocrystal below a certain critical size is an efficient photo-catalyst.
We report first-principle calculations that reveal distinct roles played by several different aspects of the nanoscale. On flat stoichiometric CdSe surfaces a CO2 molecule physisorbs and is no more reactive than in the freestate. At a Cd vacancy, however, strong chemisorptions occur, and the molecule draws extra electron density from the back bonds to become negatively charged. The barrier for desorption is ~ 0.3 eV suggesting that, even at room temperature, CO2 molecules would be constantly chemisorbing and desorbing. If a chemisorbed molecule could desorbs and carry an extra electron with it, it would be highly reactive. Photo excitation, which excites electrons to the conduction bands, is essential for the catalytic process to occur. Doping the crystal n-type in the calculation reduces the energy cost to only 0.4 eV it is now that a nano-crystal enters the scene as an absolute necessity. The energy gap of a nano-crystal increases with decreasing size. The critical diameter to enable the free flow of crystal electrons to desorbing CO2 molecules is estimated at about 3.5 nm, which compares well with the experimental value of 5 nm.
Catalyst Used for CO2 Reduction
The mechanism of CO2 reduction is followed by absorption and adsorption processes. Catalysts are loaded on high surface area porous support materials such as activated aluminium oxide and activated charcoal for CO2 reduction application. Poly ethylene glycol is used as a binder to load the catalyst on the support materials.

Activated Alumina Sphere

Activated Zeolite Pellet

Activated Charcoal Granule
Catalytic Reactor Chamber
CO2 reduction measurement is carriedout at relatively low temperature, mild steel pipe is used forthe reactor chamber. The chamber is fitted with flanges welded along the pipe on both ends. Wire mesh is mounted in the front and rear faces of the catalyst bed to hold the catalyst and to avoidabrasion of the spheres and granules. The reactor chamber is tightly packed with the catalyzed support material. The wiremesh on both ends prevents material escaping out of the catalytic reactor chamber. The catalytic reactor chamber is installed at the tail pipe of the engine after the muffler.
CO2 Reduction in Diesel Engine

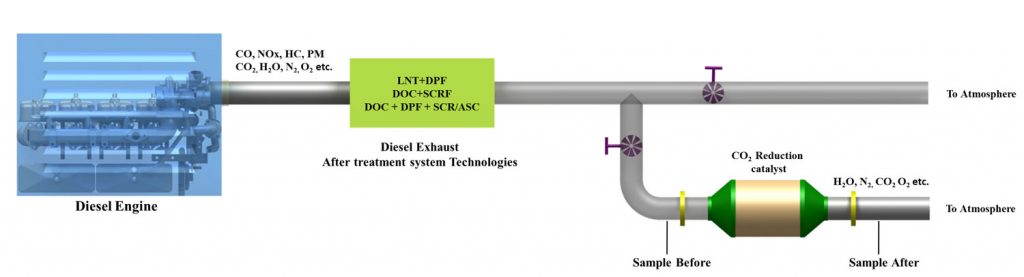
The diesel engine out exhaust is passed through after treatment system to remove CO, NOx, HC and PM. Particulate matter (PM) is trapped using Diesel Particulate Filter (DPF). The products coming out of the exhaust after treatment system contains CO2, H2O, N2 and O2 etc. This gas stream is then passed through a fixed bed CO2 catalytic reaction chamber.
CO2 Reduction in Vehicle

The gasoline-burnt internal combustion engines emit regulated gases such as CO, NOx, and HC. They are converted into CO2, H2O, and N2 by three-way catalytic converter (TWC).
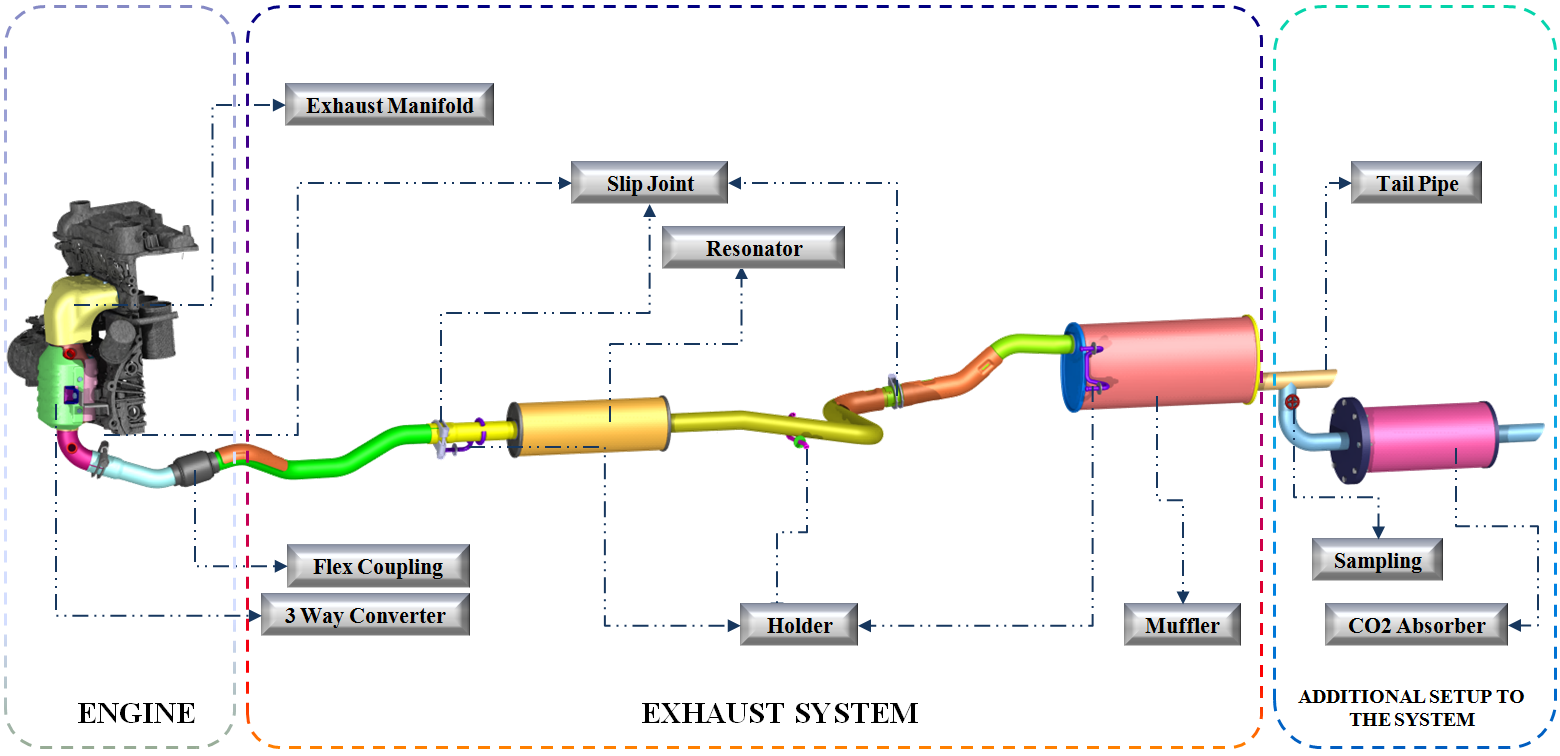
The products coming out of the three-way catalyst system contain CO2, H2O, N2, O2, etc. The slip stream of the exhaust gas coming out of the TWC, rich in CO2, is then passed through a fixed-bed CO2 absorption/adsorption catalyst chamber. Heterogeneous catalytic absorption/adsorption is carried out in a tubular reactor of one type or another. Fixed-bed tubular reactor and tubular honeycomb structures are used in the current study. The materials used for CO2 reduction is chosen to have the absorption and adsorption characteristics. Catalysts are usually loaded on high surface area porous support materials. Charcoal, alumina and ZSM-5 zeolite are few materials supports used in our current study.
Conclusion

The reduction of carbon dioxide level in the atmosphere is an immediate need as CO2 plays a significant role in the enhanced green house effect leading to abnormal climate change. The production of CO2 is increased due to heavy use of fossil fuel and industrialization. The reduction of CO2 is significantly reduced due to deforestation. The gap between the production and consumption of CO2 concentration in the atmosphere is increased in time. Natural fixation of CO2 in the atmosphere such as photo synthesis and ocean carbon cycle is enough to control the growing amount of CO2. Artificial fixation of CO2 is necessary. Carbon dioxide is captured and extracted by various methods. Techniques using effective absorber, conventional and nano-catalytic methods, capturing by enzymes and capturing using microalgae are few pathways to reduce CO2. CO2 is converted into methane, methanol, oxygen and bio mass using catalytic process. The continued challenge of converting CO2 into useful material and the need for the large CO2 reduction using a little amount of reducing material is a growing research.
The life that you are living now is also a dream of millions….
– Mylaudy Dr. S. Rajadurai
References

- International Journal of Science and Advanced Technology, 164-171, Vol. 2, No 5 (2012).
- International Journal of Science and Advanced Technology, 31-34, Vol. 4, No 8 (2014).
- International Journal of Innovative Science, Engineering & Technology, 376-380, Vol. 1, Issue 8 (2014).
- International Journal of Recent Development in Engineering and Technology, 1-5, Vol. 4, Issue 9 (2015).
- International, Journal of Research and Applications, Vol 6, Issue 5, (May 2016).
- Journal of Mechanical and Civil Engineering (IOSR-JMCE), Vol 13, Issue 3 (2016).
- Journal of Chemical and Pharmaceutical Research, 265-272, Vol. 8, Issue 5 (2016).
- International Journal of Current Research, Vol. 8, Issue, 05, 31109-31115, May 2016.
- Journal of Mechanical and Civil Engineering, 102-107, Vol 13, Issue 3 Ver. II (2016).
- International Journal of Innovative Research in Science, Engineering and Technology, 16734-741, Vol 5, Issue 9 (2016).